Slide 1 - Interpreting Keq
- Keq >> 1 → products favoured
- Keq << 1 → reactants favoured
- Keq ≈ 1 → significant amounts of both

Generated from prompt:
Make a presentation about Keq and equilibrium module 5 hsc syllabus new south wales
Explores dynamic equilibrium, Keq expressions (Kc vs Kp), Le Chatelier's Principle, Haber Process example, and key takeaways for NSW HSC Module 5 predictions and calculations.








Chemical Equilibrium & Constants (Keq & Ksp)
HSC Chemistry – Module 5: Equilibrium and Acid Reactions

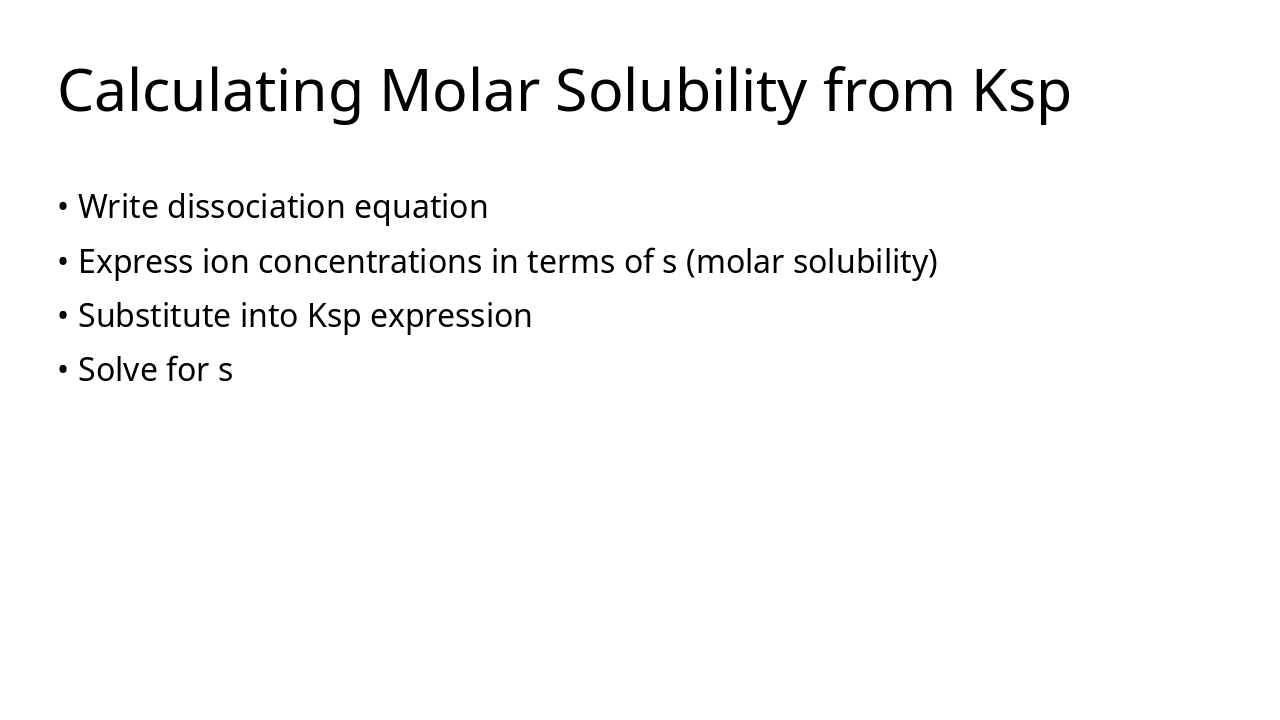
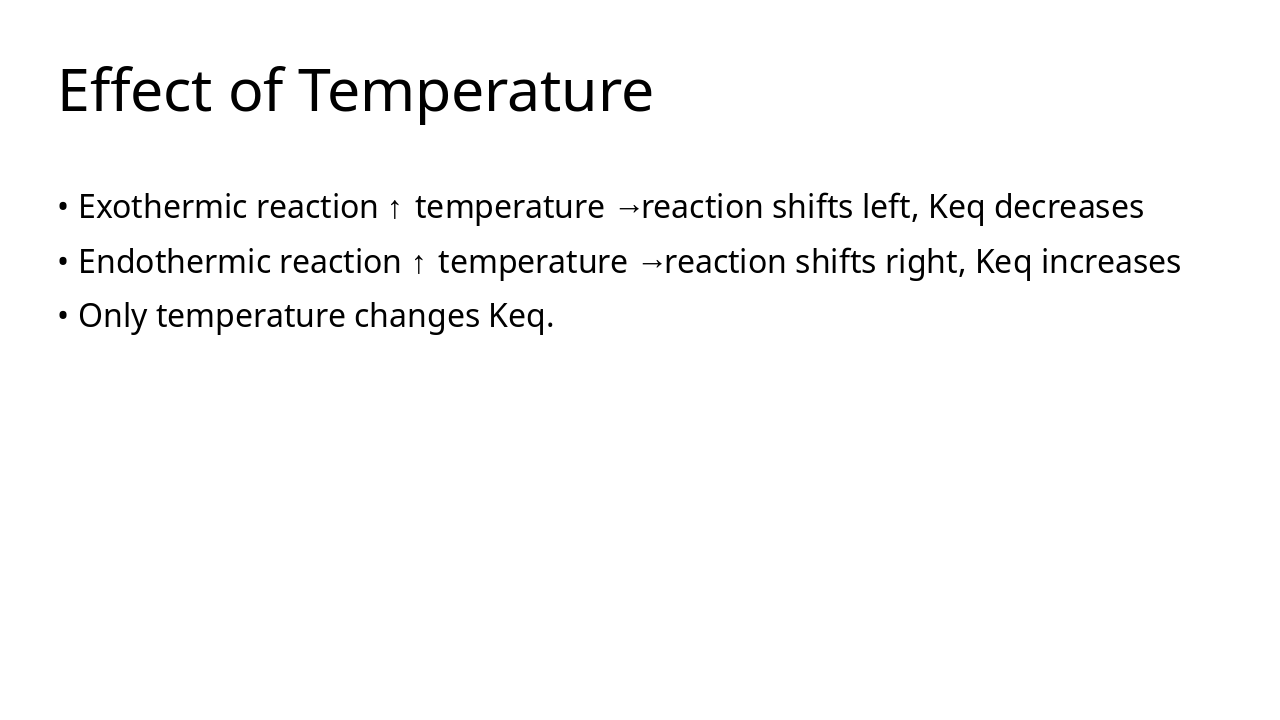
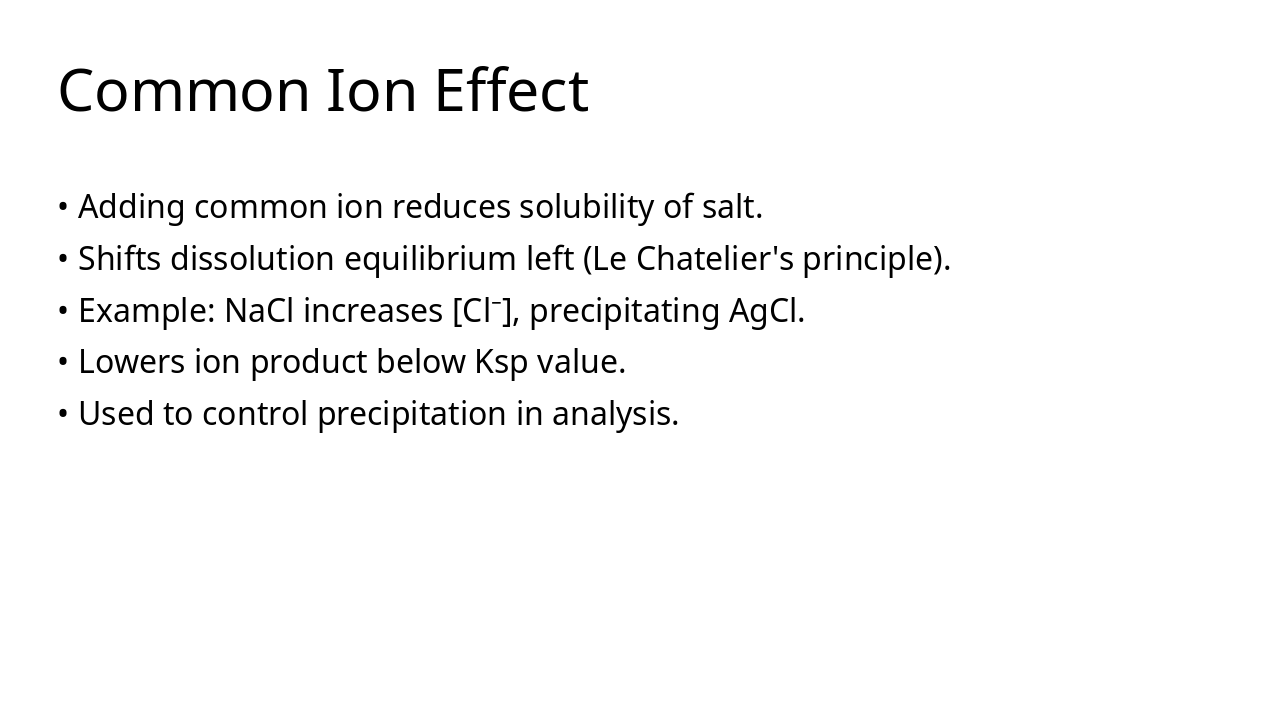
Keq quantifies equilibrium composition Temperature is the only factor that changes Keq Ksp describes solubility equilibrium Q vs Keq/Ksp predicts direction of reaction or precipitation Equilibrium principles guide industrial optimisation
Master Equilibrium for HSC Success!







Explore thousands of AI-generated presentations for inspiration
Generate professional presentations in seconds with Karaf's AI. Customize this presentation or start from scratch.