Slide 1 - Three Critical Early-Stage FDA Mistakes First-Time Medical Product Founders Must Avoid
This title slide highlights "Three Critical Early-Stage FDA Mistakes First-Time Medical Product Founders Must Avoid." Its subtitle emphasizes "Regulatory Lessons for Medical Innovators."
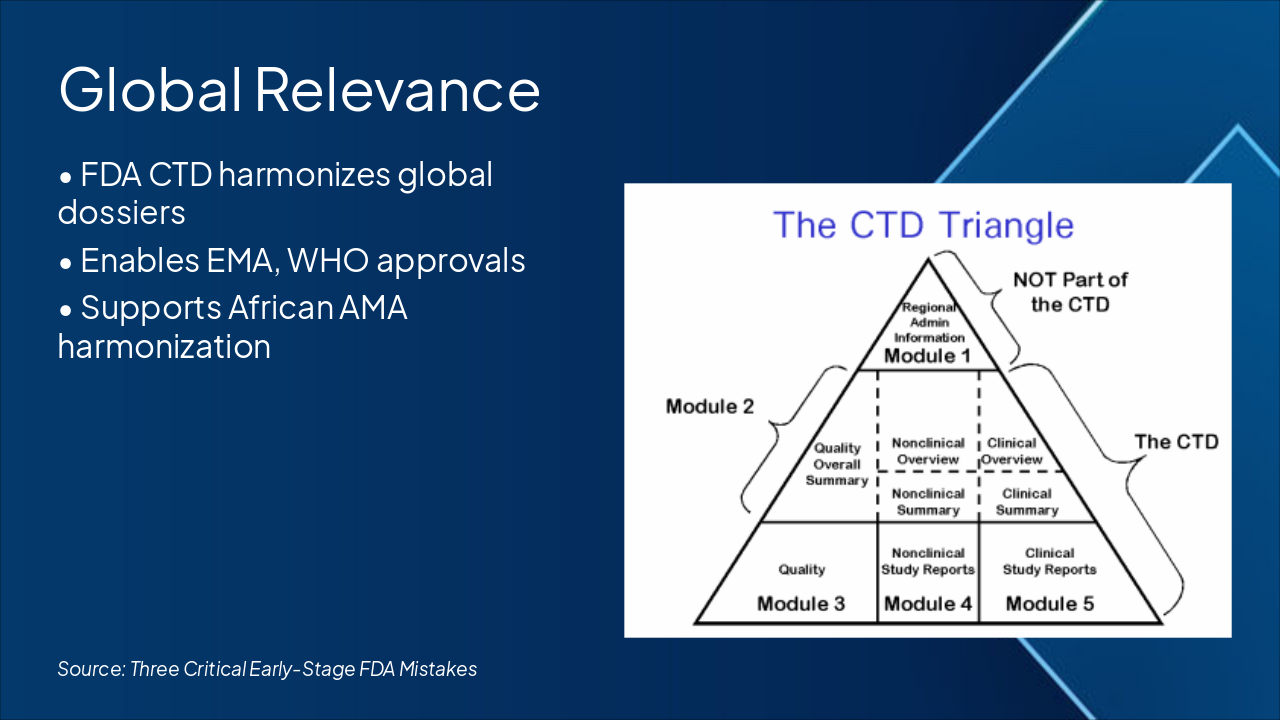
Three Critical Early-Stage FDA Mistakes
First-Time Medical Product Founders Must Avoid
Regulatory Lessons for Medical Innovators
Source: Regulatory Lessons for Medical Innovators | Author: [Your Name] | Date: [Today]